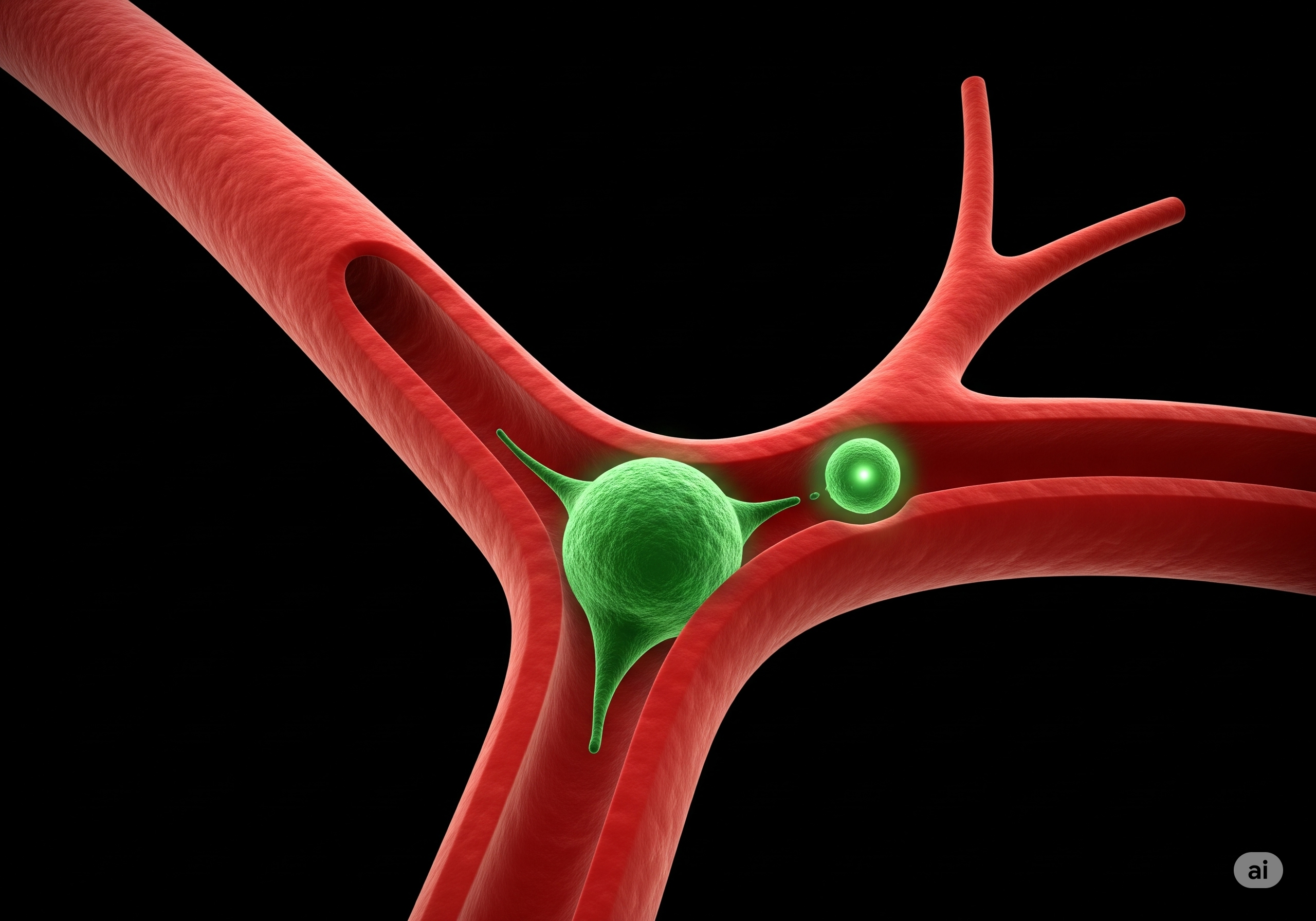
Circulating Tumour Cells (CTCs) are cancer cells that have left the primary tumour and travel to distant parts of the body where they seed new tumours. This process, known as metastasis, is responsible for 9 out of 10 cancer-related deaths but is still not fully understood. This newly published research uncovers that CTCs, as they squeeze through the body’s smallest blood vessels, shed fragments of their own cell bodies. These fragments, were identified by the team as a new type of extracellular vesicle called “shearosomes,” and appear to trigger responses in nearby capillaries and tissues that may contribute to the successful seeding of metastases.
“This is a new step forward in our understanding of metastasis. We’ve discovered that CTCs shed these shearosomes as they pass through the narrowest parts of the circulation. These vesicles aren’t just cellular debris—they actively alter the surrounding environment, damaging blood vessels and influencing immune cells to support tumour growth.”
Sam Au, Associate Professor, Bioengineering, Imperial College London and CRUK Convergence Science Centre
The team used advanced “vessel-on-chip” microfluidic systems, developed at the CRUK Convergence Science Centre Microfabrication and Prototyping Facility, to mimic the geometries of human blood vessels. This allowed them to closely monitor how tumour cells shed shearosomes under mechanical stress, and to later isolate shearosomes for further study. The team then went on to demonstrate that shearosomes damaged 3D models of blood vessel networks and induced immune cells to differentiate into cells that promote metastasis. Their findings revealed that shearosomes differ from all previously identified extracellular vesicles, both in how they are formed and in their content.
One of the study’s most surprising results came from proteomic analysis of shearosomes, led by Paul Huang’s group at the Institute of Cancer Research:
“We found that our shearosomes had far more distinct proteins than we expected, more than 3000, representing about half of the distinct proteins in parental cells. Similarly sized extracellular vesicles have previously been reported to have only a few hundred. We think this is because shearosomes are generated biomechanically by fluid shear stress, instead of other extracellular vesicles where the cells actively selects and packages their contents.”
Paul Huang, Professor, Institute of Cancer Research and CRUK Convergence Science Centre
The next steps of this research are to see to what degree shearosomes are responsible for promoting metastasis across different cancer types. In the short term, this work provides new insight into how CTCs survive and migrate through the vascular system and how they may prepare distant organs for colonisation, in line with the longstanding "seed and soil" theory of metastasis. In the longer term, the discovery of shearosomes opens new avenues for anti-metastatic therapies aimed at blocking their formation or disrupting their interactions with distant tissues.